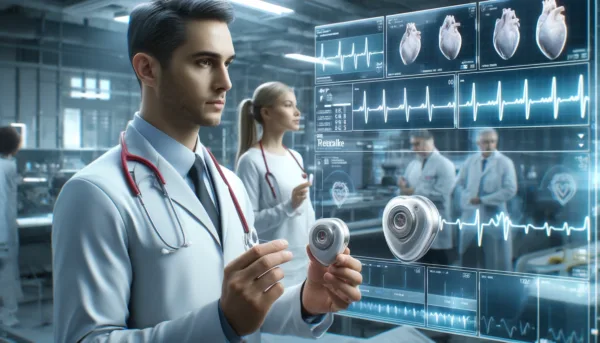
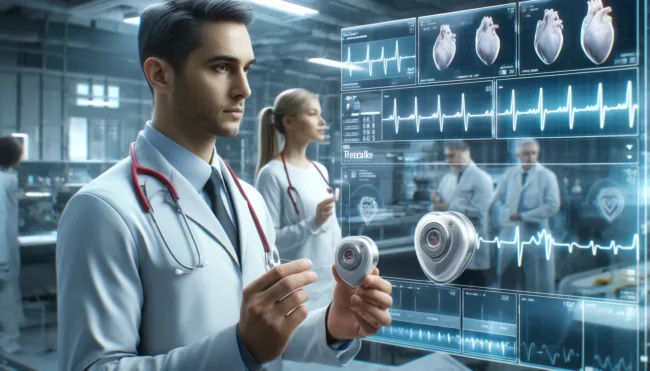
Abbott has achieved a significant milestone in cardiac healthcare by securing the CE Mark for its AVEIR dual chamber (DR) leadless pacemaker system in Europe. This groundbreaking device represents the world’s first dual chamber leadless pacemaker, specifically designed to treat individuals with abnormal or slow heart rhythms—a condition affecting approximately 49 million people across the European Union.
Pioneering Technology in Cardiac Treatment
The AVEIR DR system introduces a novel approach to dual chamber therapy, consisting of two distinct devices: the AVEIR VR for the right ventricle and the AVEIR AR for the right atrium. Each device is significantly smaller than a traditional pacemaker, roughly the size of one-tenth of these conventional devices and smaller than a AAA battery. This compact size represents a leap forward in medical technology, offering a less invasive and more discreet cardiac treatment solution.
Advantages of the Leadless Pacemaker System
Unlike traditional pacemakers, which require a surgical implantation that often results in a chest scar and visible device bulge, the AVEIR DR system is implanted directly into the heart through a minimally invasive procedure. This method eliminates the need for cardiac leads, reducing the risk of lead and infection-related complications and shortening the recovery period for patients.
Professor Reinoud Knops, M.D., Ph.D., from the Department of Cardiac Electrophysiology at the Amsterdam University Medical Center, hailed the CE Mark approval as a historic moment for European cardiac care. “As the world’s first dual chamber leadless pacemaker, AVEIR DR is a game changer, expanding our capacity to address complex heart conditions, and significantly reducing complication risks and enhancing patient comfort,” said Professor Knops.
Breakthrough i2i Communication Technology
The AVEIR DR system is equipped with Abbott’s proprietary i2i (implant-to-implant) communication technology, which enables synchronized pacing between the two leadless devices based on a patient’s clinical needs. This high-frequency pulse communication leverages the body’s natural conductivity, offering a battery-efficient alternative to the more commonly used inductive, radio-frequency, or Bluetooth communications in medical devices.
Leonard Ganz, M.D., Divisional Vice President of Medical Affairs and Chief Medical Officer at Abbott’s Cardiac Rhythm Management Business, emphasized the transformative potential of this technology. “AVEIR DR addresses a critical need for people living with slow heart rhythms and enhances people’s quality of life with its revolutionary leadless design,” he commented.
Clinical Validation and Future Prospects
The AVEIR DR i2i Global Clinical Investigation study has demonstrated the system’s effectiveness and safety, showing a 98.3% system implant success rate and maintaining atrio-ventricular synchrony in more than 97% of patients. Following its recent U.S. FDA approval in June 2023, the AVEIR DR system is poised to transform the landscape of cardiac care, offering a new, minimally invasive option for patients worldwide.
Discover more from Business-News-Today.com
Subscribe to get the latest posts sent to your email.