Novarad said that its augmented reality (AR) powered VisAR surgical navigation system has secured clearance from Indonesia’s Food and Drug Administration (FDA) for intraoperative use in stereotactic spinal surgery.
According to the American medical imaging solutions provider, VisAR is accurate for open as well as minimally invasive surgery (MISS).
Novarad said that its technology for AR surgical navigation allows surgeons to transform the patient’s image data into the form of a 3D hologram that could be projected over the body of the patient with absolute accuracy. This allows surgeons to focus exclusively on the goal of surgery without having to glance away from an additional monitor, thereby providing precise surgical guidance.
VisAR System is said to be a complete solution that incorporates pre-surgical planning and segmentation, virtual annotations as well as two-way picture connectivity. It also offers integrated 2D and 3D navigation views as well as ongoing hologram-to patient registration.
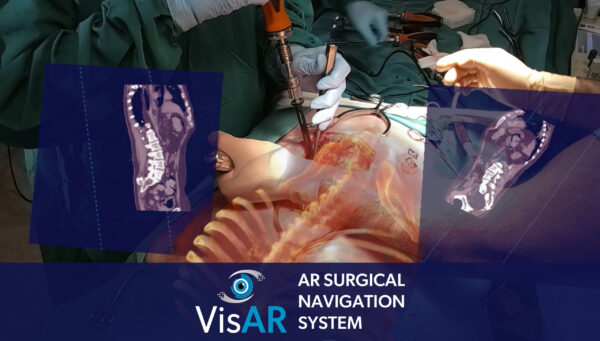
The AR surgical navigation system is able to provide a sub-2-millimeter accuracy rate for the placement of pedicle screws in minimally invasive and open surgical procedures. With a quick and easy installation, VisAR enables the surgeon to see the entire OR footprint by using voice-controlled commands.
VisAR’s technology uses CT fiducial markers that are visible in medical images for automated registration.
Novarad stated that it has partnered with Microsoft to use the pre-built AR headset technology that allows an affordable price and the capability to take advantage of the anticipated advancements in hardware.
With VisAR, doctors can use the wireless Microsoft HoloLens 2 visor and are not required to use additional navigational equipment.
The augmented reality surgical technology is claimed to create the lowest OR size of any other system on the market and is part of Novarad’s technology stack for imaging that offers connectivity, HIPAA compliance, image management, and a variety of security features.
VisAR is presently available in the US and now in Indonesia, with other countries likely to authorize the surgical navigation technology in upcoming months, said Novarad.
Dr Wendell Gibby — Novarad CEO and VisAR co-creator said: “We are thrilled to bring VisAR’s revolutionary technology to Indonesia.
“VisAR’s FDA approval in Indonesia is a testament to its innovative design and ability to improve patient outcomes. This technology will enable surgeons in Indonesia to perform complex procedures with greater accuracy and less risk, ultimately benefiting patients.”
Discover more from Business-News-Today.com
Subscribe to get the latest posts sent to your email.





