Kintor Pharmaceutical said that it has wrapped up patient enrollment for the phase 2 clinical trial of KX-826 (pyrilutamide) in China for the treatment of acne vulgaris.
The Chinese biotech company has enrolled a total of 160 patients for the mid-stage clinical trial.
According to Kintor Pharmaceutical, the randomized, double-blind, placebo-controlled clinical study to be held in multiple regions will assess the safety and efficacy of KX-826 in gel form in patients having mild to moderate acne vulgaris.
The primary endpoint of the KX-826 phase 2 clinical trial is the treatment success rate in each group at the end of week 12 as per the five-point Investigator Global Assessment (IGA) scale. Subjects whose IGA scale is reduced by not less than two levels to 0-1 are defined as success.
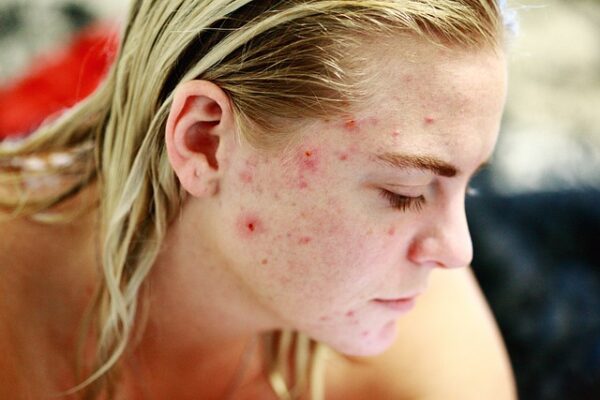
Dr. Youzhi Tong — Kintor Pharmaceutical founder, chairman, and CEO said: “KX-826’s clinical trial in China for treating acne vulgaris was the combined design of phase I and phase II clinical trials. KX-826’s phase I clinical trial has demonstrated a preliminary positive safety and tolerability profile in terms of dose-escalation and multiple topical doses applications per day for local use.
“We expect that KX-826 continues to demonstrate good efficacy and safety in phase II clinical trial to benefit more people suffering from acne vulgaris.”
KX-826 is an androgen receptor (AR antagonist) which has potential for being a topical drug for treating androgenetic alopecia (AGA) and acne vulgaris, said the Chinese biotech company.
Discover more from Business-News-Today.com
Subscribe to get the latest posts sent to your email.






