Abiomed gets FDA nod for Impella ECP pivotal heart pump in clinical trial
The US Food and Drug Administration (FDA) has cleared Abiomed to use the company’s version of Impella ECP pivotal heart pump in pivotal clinical trial.
The Impella ECP pivotal clinical trial is a single-arm, prospective, and multi-center trial and has enrolled the first two patients.
The clinical trial will assess the rate of major adverse cardiovascular and cerebrovascular events in individuals who receive Impella ECP support during an elective or urgent high-risk percutaneous coronary intervention (PCI).
Impella ECP is said to be the world’s smallest heart pump and the only heart pump compatible with small bore access and closure techniques.
The heart pump is 9 French in diameter upon insertion and removal from the body and provides peak flows up to 5 L/min on insertion into the body to expand and support the heart’s pumping function.
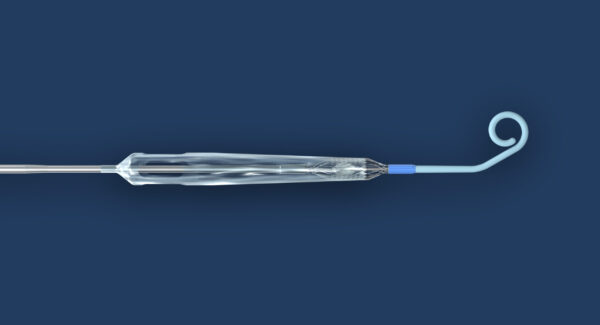
Abiomed gets FDA nod for Impella ECP pivotal heart pump in clinical trial. Photo courtesy of Business Wire.
Of the two patients who received Impella ECP support during challenging left main coronary bifurcation stent procedures involving heavily calcified lesions, the first patient was closed with an 8 Fr closure device following Impella ECP removal.
Dr Amir Kaki — Impella ECP FDA pivotal trial national principal investigator said: “Impella ECP advances the opportunity for physicians to provide critical hemodynamic support during high-risk PCI procedures by delivering similar or higher flow compared to other options through a smaller vascular sheath for access.
“This technology has the potential to improve patient safety and cath lab throughput because of the smaller arteriotomy required for pump placement.”
In March 2022, The Impella ECP Pivotal Clinical Trial received FDA approval to enroll up to 217 patients in the US.
In August 2021, Impella ECP received FDA breakthrough device designation.
Discover more from Business-News-Today.com
Subscribe to get the latest posts sent to your email.

