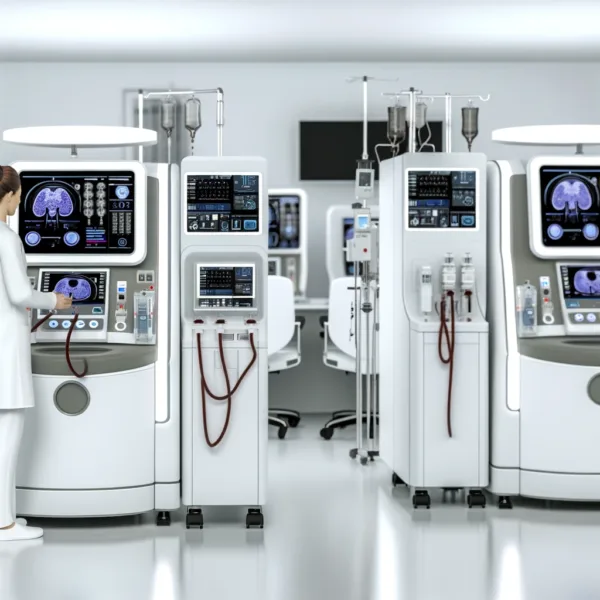
Outset Medical, Inc., a leader in innovative medical technology, has announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its new product, TabloCart with prefiltration. This accessory is designed to enhance the functionality of the Tablo Hemodialysis System, already known for its revolutionary approach to dialysis by reducing the procedure’s cost and complexity.
Enhancing Dialysis Care with Flexible Solutions
Leslie Trigg, Chair and CEO of Outset Medical, highlighted the significance of this clearance: “With the FDA’s clearance of TabloCart with prefiltration, we have delivered features to the Tablo Hemodialysis System that increase the flexibility for nurses and health systems to serve patients from the ICU to the bedside,” she stated. “Today’s announcement underscores Outset Medical’s commitment to innovation and our ongoing dedication to transforming the landscape of dialysis treatment.”
The TabloCart offers two versions—one with added storage and another featuring water prefiltration capabilities—to meet diverse clinical needs. The prefiltration version is especially critical as it allows for adaptation to varying water conditions, enhancing the system’s usability across different settings.
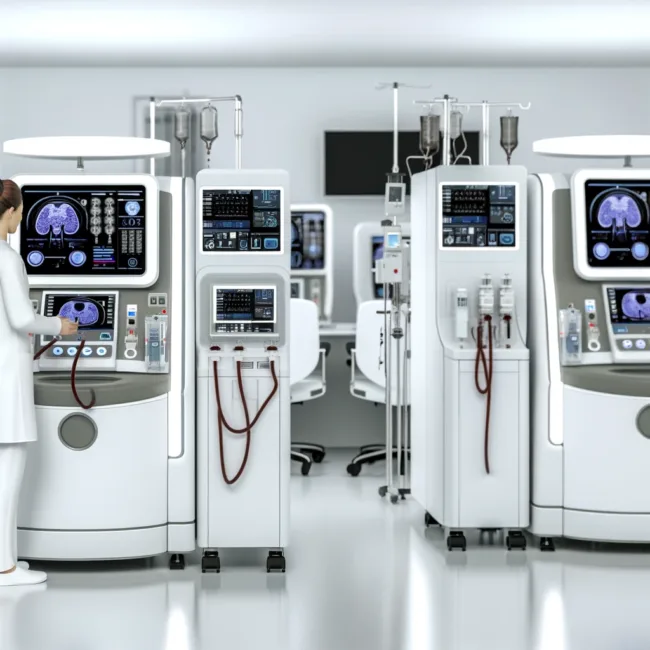
Key Features of the New TabloCart
TabloCart’s design focuses on operational efficiency and user-friendly features, including:
– Versatile Prefiltration Options: It offers three customizable prefiltration settings, catering to various water conditions, and includes a booster pump to increase incoming water pressure.
– Enhanced Maneuverability: The cart is equipped with 360-degree rotating wheels and directional locking rear wheels, which facilitate easy movement throughout healthcare facilities.
Transforming Dialysis Treatment Across the Care Continuum
Tablo, used from the hospital to the home, simplifies the dialysis experience for patients and streamlines operations for providers. It requires only access to tap water and a standard electrical outlet, positioning Tablo as a single enterprise solution usable across the continuum of care. This flexibility allows for dialysis to be delivered anytime, anywhere, by virtually anyone.
With this latest FDA clearance, Outset Medical resumes distribution of TabloCart with prefiltration in the United States, with products now available to ship to customers.
The approval of TabloCart with prefiltration marks a significant milestone in Outset Medical’s journey to enhance dialysis treatment through technology. This development promises to improve the quality of life for dialysis patients by offering more adaptable and convenient treatment options.
Discover more from Business-News-Today.com
Subscribe to get the latest posts sent to your email.